Van Der Waals Forces Examples
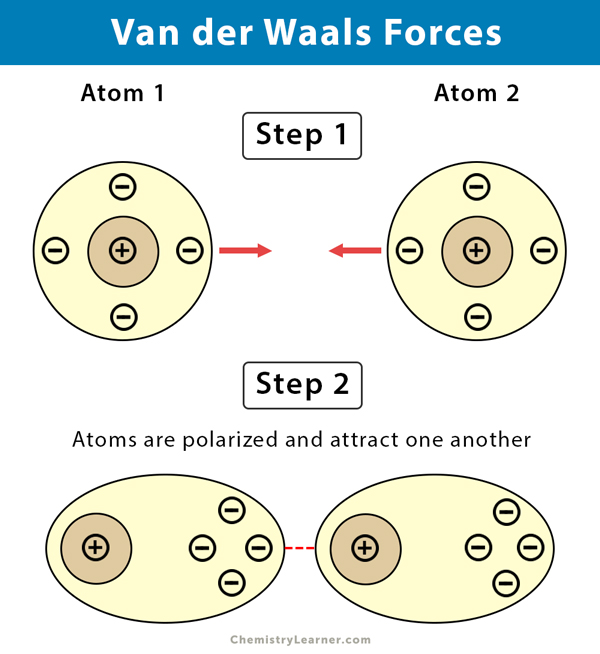
Van der Waals forces is a general term used to define the attraction of intermolecular forces between molecules. The word a in the Waals equation shows the intensity of attraction among molecules or atoms.

Van Der Waals Forces Definition Overview Expii
The firmness of a.

. The influence of the van der Waals forces can be illustrated using the example of alkanes. What are the types of van der Waals forces. Van der Waals forces are always present while electrostatic forces are crucial only in non-neutral or.
Electrostatic induction and dispersion. Examples Van der Waals forces in alkanes. Examples of Van der Waals forces in the following topics.
Van der Waals forces depend on the molecular surface area. Van der Waals forces. For example the boiling points of pentane and hexane are 36ᵒC and 69.
Examples of van der Waals forces include hydrogen bonding dispersion forces and dipole-dipole interactions. Examples of van der Waals forces include hydrogen bonding dispersion forces and dipole-dipole interactions. The Van Der Waals equation studies the properties of two gases.
There are two kinds of Van der Waals forces. Van der Waals forces may be classified into three types. Dependence of Van Der Waals Forces.
The weaker van der Waal force works only within a short range. Examples of van der Waals Force. What is a real world example of van der Waals forces.
However they may assist a necessary. Why are van der Waals interactions important. A perfect example of this is table salt NaCl which has a melting point of 800 degrees Celsius.
For example consider London dispersion forces between two chlorine molecules. Physical adsorption include Van der Waals interactions and electrostatic attractions. London dispersion forces are part of the van der Waals forces or weak intermolecular attractions.
Ion interactions are stronger and are classified separately from the weaker van Der Waal interactions. Here both chlorine atoms are bonded through a covalent bond which forms by equal sharing of valence. The carbon layers are neatly.
The Van der Waals force between atoms molecules and surfaces is a part of everyday life in many different ways. Like hydrogen bonds van der. Van Der Waalss forces of attraction are the weakest among the weak chemical forces having a strength that ranges between 04 and 4-kilo Joulesmol.
Is Van der Waals a covalent bond. Graphite a form of Carbon seen in a Pencil is one of the common examples where one observes van der Waals force. Van der Walls force.
Types of intermolecular forces Van Der Waals forces Van der Waals forces aka Van der Waals.


0 Response to "Van Der Waals Forces Examples"
Post a Comment